Abstract
Introduction: Iron deficiency is the most frequent cause of anemia, which afflicts 32.9% of the global population and is a significant cause of morbidity worldwide. Oral iron is the first-line therapy for treating iron deficiency anemia; intravenous(IV) iron is used when oral iron is inappropriate, poorly tolerated or ineffective. The major adverse effect associated with IV iron is infusion-related reactions (IRR), ranging from mild reactions mediated by complement activation-related pseudo-allergy to anaphylaxis. In this study, we sought to determine rates and severity of iron IRR, identify patient characteristics associated with IRR and report strategies for subsequent iron repletion in patients who have IRR.
Methods:
We conducted a retrospective chart review of all patients older than 18 years who received IV iron from 1/1/2016 to 7/20/2021 in the outpatient Classical Hematology clinic at Yale New Haven Hospital. Demographics, risk factors, details of reactions and follow up were noted. The primary outcomes analyzed included rates and severity of IRR based on nursing and provider documentation and graded according to a previously-published IRR severity classification (Rampton D et al, Haematologica 2014;99:1671). Secondary outcomes included identification of patient characteristics associated with IRR utilizing Chi-square or Fisher's exact test for unadjusted analysis and logistic regression for multivariable analysis (IBM SPSS version 28).
Results:
A total of 330 patients, including 298 females, received IV iron. The common formulations used were ferumoxytol, iron sucrose and iron dextran with 199 (60.3%), 49(14.8%), and 36 (10.9%) patients, respectively, receiving each of these. Comorbid conditions were present in 317 (96.1%) patients, including 114 (34.5%) with cardiovascular disease (CVS) and 71 (21.5%) with asthma. Beta blocker or angiotensin-converting enzyme inhibitor (ACEI) use was noted in 90 (27.3%) patients. A history of allergies to medications was present in 167 (50.6%) patients while 139 (42.1%) had other non-drug allergies. The common indications for infusion were anemia due to heavy menstrual bleeding (27.6%), gastrointestinal bleeding (17.2%) and pregnancy (6.9%).
Iron IRR was noted in 58 (17.6%) patients, of whom 36 (62.1%) had previously tolerated IV iron. Of the 58 patients with IRR, the median age was 40.32 ± 14.6 years; 30 (51.7%) had allergies to medications, 34 (58.6%) had non-drug allergies, and 13 (22.4%) each had a history of CVS or asthma. Beta blocker or ACEI use was found in 12 (20.7%) patients. The reaction manifestations included gastrointestinal in 16 (27.6%) patients, cardiovascular in 24 (41.4%), bronchopulmonary in 34(58.6%), mucocutaneous in 28(48.3%), neurological in 21(36.2%) and autonomic in 30 (51.7%). The severity of reaction was mild in 22 (37.9%) patients, moderate in 23 (39.7%), and severe in 11 (19%). After a reaction, 28 (48.3%) patients subsequently tolerated a different formulation of IV iron, while 14 (24.1%) patients tolerated the same formulation of IV iron at a slower rate with premedication; 5 (8.6%) patients had a subsequent reaction to a different IV iron formulation.
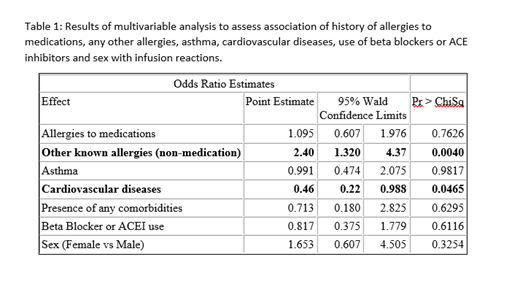
Multivariable regression analysis was performed to examine associations of iron IRR with history of allergies to medications, non-drug allergies, asthma, CVS, use of beta blockers or ACEI and sex. In this analysis, a history of non-drug allergies was significantly and independently associated with greater odds of IRR (odds ratio (OR) 2.40, 95% confidence interval (CI): 1.32-4.37, p=0.004) while a history of CVS was associated with lower odds of IRR (OR 0.46, 95% CI: 0.22-0.988, p=0.047). None of the parameters were found to have an association with the severity of IRR.
Conclusion:
Iron IRR occurred in 17.6% of patients receiving IV iron, with severe reactions occurring in 3.3% of all patients; these rates are higher than those previously reported in literature. A history of non-drug allergies is independently associated with greater odds of IRR, and additional precautions may be indicated to prevent IRR in such patients. An association of CVS with lower odds of reaction may reflect anti-inflammatory effects of widespread aspirin use in these patients. The majority of patients with iron IRR can subsequently tolerate the same or a different formulation of IV iron with slower rates of infusion and premedication.
Neparidze: Janssen: Research Funding; GlaxoSmithKline: Research Funding; Eidos Therapeutics: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal